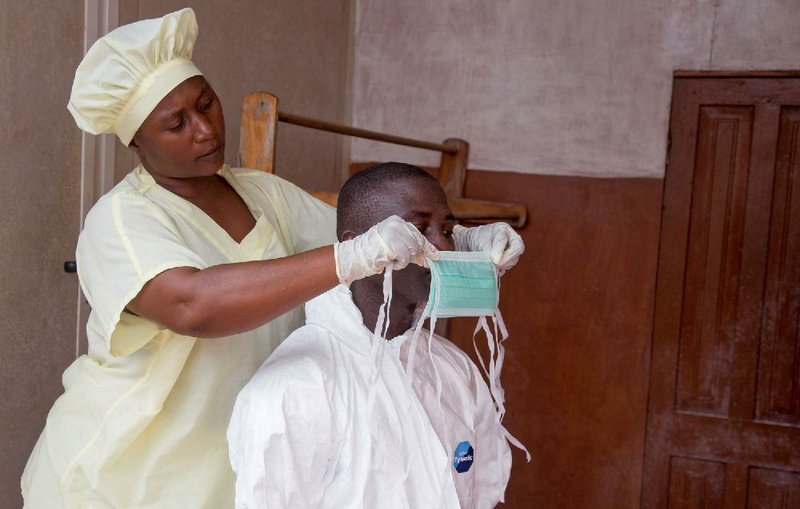
DAKAR, Senegal -- Doctors treating a Sierra Leone physician who had Ebola defended their decision not to give him an experimental drug, saying Wednesday that they feared it was too risky.
Calling it "an impossible dilemma," Doctors Without Borders explained in detail its decision in response to a New York Times story on the case.
The explanation came the same day that another top doctor from Sierra Leone died of the disease, further fueling a debate about how to apportion a limited supply of untested drugs and vaccines and whether they are even effective.
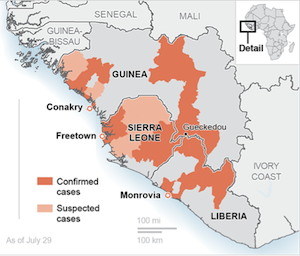
Ebola has killed more than 1,000 people and sickened nearly 2,000 in the current outbreak, which also has hit Guinea, Liberia and Nigeria. Many of the dead are health workers, who often work with inadequate supplies and protection.
At the time the experimental treatment was being considered for Dr. Sheik Humarr Khan, his immune system was already starting to produce antibodies suggesting he might recover, Doctors Without Borders said in the statement. Khan also was due to be transferred to a European hospital that would be more capable of caring for him, it said.
The experimental drug, ZMapp, is designed to boost the immune system to help it fight the virus.
In the end, the treating physicians decided against using the drug. They never told Khan of its existence because they felt it would be unethical to tell him of a treatment they might not use. Shortly after their decision, however, Khan's condition worsened, the statement said, and the company providing the medical evacuation decided not to transfer him. He died a few days later, on July 29.
"Every day, doctors have to make choices, sometimes difficult, about treatment for their patients," said the Doctors Without Borders statement. "Trying an untested drug on patients is a very difficult decision, particularly in the light of the 'do no harm' principle."
ZMapp has since been used on two Americans and a Spaniard. The California-based company that makes the drug, Mapp Pharmaceuticals, has said its supplies are now exhausted, and it will take months to produce even a modest amount.
The drug had never before been tested in humans, and it is not clear if it is effective or even harmful. The Americans are improving -- although it is unclear what role ZMapp has played in that -- but the Spaniard died Tuesday.
The last known doses of ZMapp arrived Wednesday in Liberia, carried personally by Foreign Minister Augustine Ngafuan.
Dr. Moses Massaquoi, who helped the Liberian government acquire it, told reporters at the airport that there was enough to treat three people. Previously, the government had said it would only have enough to treat two sick doctors.
They would be the first Africans known to receive the treatment.
Meanwhile, Canada has promised to donate 800 to 1,000 doses of its untested Ebola vaccine to the World Health Organization, and already questions are being asked about who will get it and how scientists will determine if it works.
Likely candidates for the vaccine are health care workers in Africa who are among the most vulnerable because of their close contact with Ebola patients.
Massaquoi said negotiations for access to the vaccine in Liberia are taking place now. Guinea also is considering asking for it.
Unlike ZMapp, which is being given to only a handful of people and is unlikely to yield significant information about the drug's effectiveness, the vaccine could be tested in a small, but more rigorous field trial.
"It gives us an opportunity to test the vaccine in an outbreak situation in populations that are at risk," said David Heymann, who professor at London School of Hygiene and Tropical Medicine.
Meanwhile, Nigeria confirmed that another person died from Ebola, raising the toll in that country to three. The man was under quarantine because he had contact with Patrick Sawyer, a Liberian-American who flew into Nigeria with the disease and died of it last month.
Information for this article was contributed by Clarence Roy-Macaulay, Jonathan Paye-Layleh, Wade Williams, Boubacar Diallo and Maram Mazen of The Associated Press.
A Section on 08/14/2014