GENEVA -- The World Health Organization on Tuesday endorsed the use of untested drugs to fight the Ebola virus, hours after a Spanish priest who had been supplied with experimental medication became the first European to die in the world's worst known outbreak of the disease.
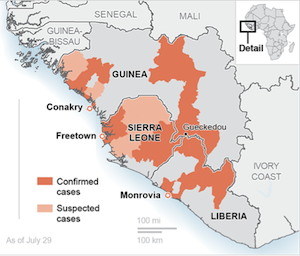
No proven cure or vaccine exists for the Ebola virus, which the organization said has killed at least 1,013 people in four West African countries -- Guinea, Liberia, Nigeria and Sierra Leone. About half of the people infected in the outbreak, first reported in March, have died. Last week, the WHO declared the outbreak a global health emergency.
The Spanish priest, Miguel Pajares, 75, worked in a hospital in Liberia and was the first European to return home after being infected with Ebola.
Citing medical confidentiality rules, hospital officials in Madrid declined to say whether the priest had ultimately been treated with the experimental drug, ZMapp, made in the United States, but the Spanish Health Ministry said Monday that it had obtained the medication for him.
Two U.S. aid workers, Dr. Kent Brantly and Nancy Writebol, who were evacuated to Emory University Hospital in Atlanta, received the drug as well. Family members said Tuesday that Writebol was getting better. Her son, Jeremy Writebol, said doctors expect her to recover.
The supplying of ZMapp, a previously untested drug in extremely limited supply, to foreign aid workers evacuated from West Africa has raised broad ethical questions about the disparities in treatment between white outsiders and the Africans who form the overwhelming majority of the victims.
Tuesday the Liberian government announced that it would receive ZMapp after a request from President Ellen Johnson Sirleaf. It said the drug would be used to treat two doctors battling for their lives against the Ebola virus. That would be the first known use of the drug to treat Africans.
The manufacturer of the drug, Mapp Biopharmaceutical, said it had complied with a request received over the weekend from a West African nation, though it noted in a statement that the available supply of ZMapp was now "exhausted."
In Geneva, the WHO convened an ethics panel Monday to debate the broader use of untested drugs. In a statement on its website Tuesday, it said that given "the particular circumstances of this outbreak," the panel had reached a consensus that "it is ethical to offer unproven interventions with as yet-unknown efficacy and adverse effects, as potential treatment or prevention."
The panel said the use of untested drugs should be guided by ethical criteria, including transparency about all aspects of the care provided, informed consent of the patient, freedom of choice and patient confidentiality.
Canada's health minister, Rona Ambrose, said after the WHO's decision was announced that Canada will donate 800-1,000 doses of an untested Ebola drug to the organization. Canada will keep a small supply of the experimental vaccine in case it is needed for use there.
WHO officials said another meeting would be held at the end of the month to address how to allocate scarce treatments.
Dr. Marie-Paule Kieny, assistant director-general of the WHO, said at a news conference in Geneva on Tuesday that there were several drugs and vaccines that have shown some promise in animal testing that might conceivably be deployed in the outbreak.
However, she said, none "is available in unlimited supplies right now." She added, "I don't think that there could be any fair distribution of something which is available in such a small quantity."
The Spanish priest, Pajares, was flown back to Spain on Thursday with a nun who worked with him, Juliana Bohi, who has tested negative for the Ebola virus. Pajares' death Tuesday from the Ebola virus was the first reported on European soil.
Spanish authorities said they had obtained a supply of ZMapp last week, without giving details.
On Monday, Jose Maria Viadero, the director of Juan Ciudad, a nongovernmental organization linked to the religious order Pajares belonged to, said Pajares was "stable" and had been treated with the experimental drug ZMapp since the weekend.
The hospital and Spain's Health Ministry, however, would not comment Tuesday about how Pajares had been treated, nor did they offer any assessment of the efficacy of the ZMapp experimental drug.
Mapp Biopharmaceutical said it had complied with every request for the drug that had the necessary legal and regulatory approvals.
Mapp and the U.S. government, which has financed most of the company's work, are making plans to increase supplies of ZMapp. But it is expected to take several months to increase supplies, and even then, there may be no more than a few hundred doses available, and perhaps less, according to federal officials and corporate executives.
Information for this article was contributed by Andrew Pollack and Raphael Minder of The New York Times and by Rob Gillies and staff members of The Associated Press.
A Section on 08/13/2014